COVID-19: Siemens’ assay outperforms antibody tests from competitors
In a Public England Health study, Siemens Healthineers' COV2T antibody test was the only assay found to meet both sensitivity and specificity requirements of the MHRA.
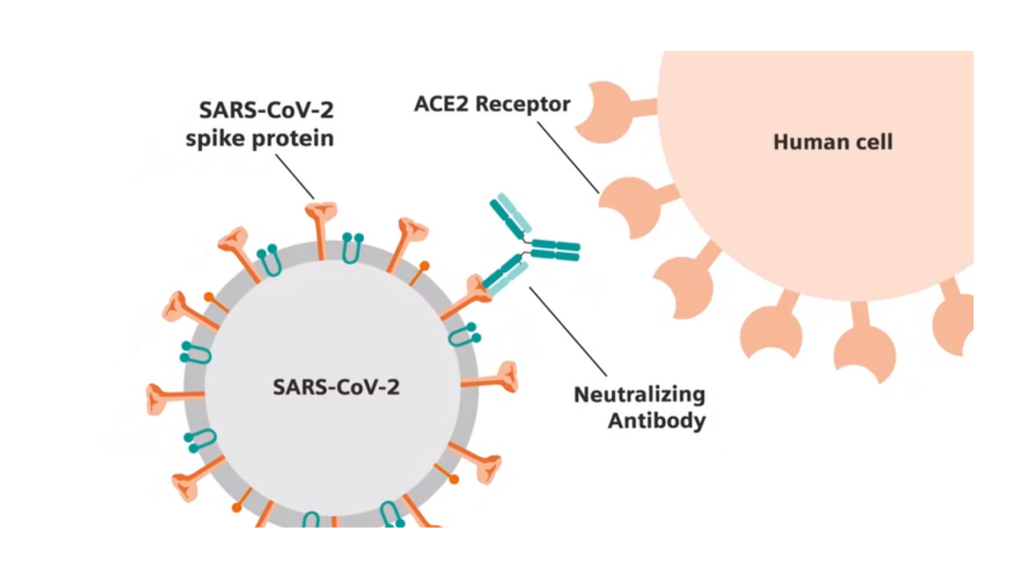
For Germany’s government, it is bad news: to establish immunity passports aimed at identifying people who have developed antibodies against SARS-CoV-2, the new coronavirus strain, Health Minister Jens Spahn ordered 3 million of Roche Diagnostics’ Elecsys® Anti-SARS-CoV-2 tests in May and 5 million tests per month from June onwards. Now, an independent Public Health England study found that Siemens Healthineers’ COVID-19 antibody test outperformed not only Roche’s but also those developed by Abbott and Diasorin. The COV2T antibody test from Siemens Healthineers was the only assay found to meet both sensitivity and specificity requirements as set out in the MHRA’s Target Product Profile (TPP) for enzyme immunoassays.
The head-to-head evaluation comprised of approximately 1,000 pre-pandemic (negative) and over 500 positive convalescent samples from individuals confirmed to have had SARS-CoV-2 infection. For Siemens, however, the evaluation might have no impact as Juliet Bryant from the Fondation Mérieux in Lyon (France) and colleagues published, in mid-May, evidence that COVID-19 antibody tests, which detect the spike- and/or nucleocapsid antigens of SARS-CoV-2, inadequately assess individual immunity to the novel coronavirus.
Published in the peer-reviewed journal Science Immunology (doi: 10.1126/sciimmunol.abc6347), the study was bad news for policymakers looking to relax social distancing measures with the help of immunity passports’. The researchers did say, however, that such tests could provide valuable information when it comes to addressing epidemiological questions like when to relax stay-at-home orders or school closures.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink